What if the “final frontier” isn’t space—but rather the three pounds of wetware sitting between your ears?
For decades, the brain was a black box. We could drug it or scan it, but we couldn’t really talk to it. That’s changing. Neurotechnology (Neurotech) is the science and business of interfacing directly with the human nervous system.
Biology meets silicon.
This is convergence driven by two massive waves crashing together: a “Silver Tsunami” of aging populations facing a surge of neurological issues like Alzheimer’s and Parkinson’s, and AI breakthroughs that can finally decode the chaotic noise of neural signals into actionable data.
The sector tells a story of three distinct approaches working in tandem.
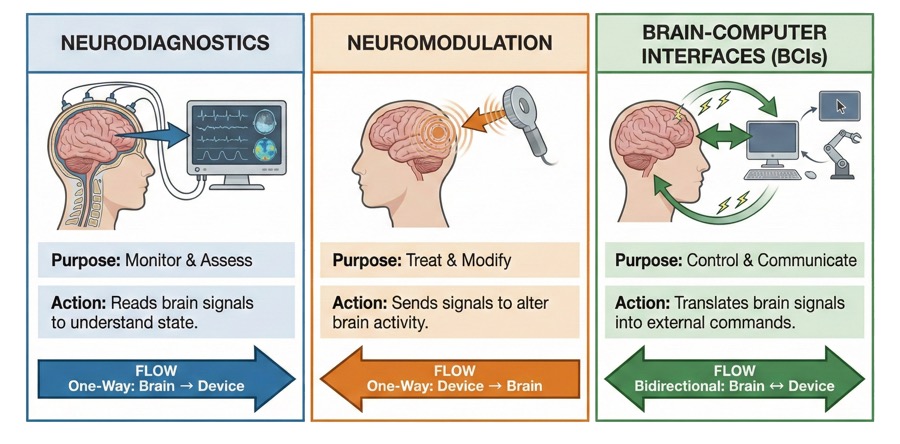
- Neuromodulation & Neurostimulation: This is the beachhead of revenue. Instead of using pills, these technologies use precise electrical pulses to alter nerve activity. Think of it as a pacemaker for the nervous system, treating chronic pain, epilepsy, and tremors with FDA-approved devices.
- Neurodiagnostics & Monitoring: Before we can fix the brain, we have to map it. This is the “check engine light” of the sector. Companies here utilize advanced EEGs, eye-tracking, and AI-driven biomarkers to detect everything from concussions to dementia years before symptoms appear.
- Brain-Computer Interfaces (BCI) & Neural Implants: This is the “moonshot” growth engine. Headlines focus on Elon Musk’s Neuralink, but the field is broader. The goal here is connection: bridging the gap between the biological brain and digital devices to restore function to paralyzed limbs or allow direct mental control of computers.
Of course, this sector is still a regulatory minefield where companies live or die by the binary outcome of FDA trials. Unlike a software app that can be patched overnight, a brain implant is forever, meaning the barrier to entry is incredibly high and the timeline to profitability is long.
We are essentially in the “dial-up internet” phase of neurotech—clunky and early, but with a clear line of sight to a future where we rewire the human experience. Investors who can stomach the volatility have a chance to buy into the ground floor of potentially the next great leap in human evolution.
Neuromodulation & Neurostimulation
For much of the last century, medicine has been dominated by chemistry. If you had a problem, you swallowed a molecule (a pill) that flooded your entire system just to reach one specific target. It’s like carpet-bombing a city to take out one building. The side effects—nausea, weight gain, lethargy—are the collateral damage.
Neuromodulation flips the script and focuses on electricity instead.
Your nervous system is essentially a biological wire harness. Neuromodulation devices tap into those wires to deliver a precise electrical “dose” exactly where it’s needed, and nowhere else. It is the difference between a carpet bomb and a sniper shot.
This is the most mature, revenue-stable corner of neurotech stocks. These companies sell “pacemakers” for the brain, lungs, and nerves that are already FDA-approved and reimbursed by insurance.
Inspire Medical Systems (NYSE: INSP)
Inspire is the company that is finally killing the CPAP machine. For decades, the only answer for Sleep Apnea was strapping a loud, uncomfortable air hose to your face every night. Compliance was terrible; half of patients eventually just threw the machine in the closet.
Inspire built a tiny device that is implanted like a pacemaker. It monitors your breathing while you sleep and delivers a gentle pulse to the hypoglossal nerve, which pushes your tongue forward and opens your airway. No hose, no mask, no noise.
The 2026 Catalyst: Inspire V’s Rollout. The narrative here is changing from “adoption” to “operational efficiency.” In 2025, Inspire launched their next-generation device, the Inspire V. Unlike the old version, this unit eliminates the need for a separate sensing lead, reducing the procedure time for surgeons. With the new CPT reimbursement codes now finalized, the administrative friction for hospital adoption has been removed. 2026 marks the first full year of Inspire V availability as the primary standard of care.
LivaNova PLC (NASDAQ: LIVN)
LivaNova is the “blue chip” of this basket, built on the back of its Vagus Nerve Stimulation (VNS) franchise. They have treated drug-resistant epilepsy for decades. They wrap a small lead around the Vagus nerve (the super-highway connecting the brain to the body) to stop seizures before they happen. It is a steady, established business.
But you aren’t buying LIVN for the epilepsy business alone. You are watching it for the “free option” on depression.
The 2026 Catalyst: RECOVER Study Verdict. Treatment-Resistant Depression (TRD) is the Holy Grail of neurotech. LivaNova is currently running the RECOVER clinical trial to prove their VNS device can lift depression when drugs fail. In 2026, the company is scheduled to release pivotal data from this study. This data will be the primary evidence used by CMS (Medicare) to determine if coverage policy should be reversed to include depression patients, which would significantly alter the company’s total addressable market.
NeuroPace, Inc. (NASDAQ: NPCE)
If LivaNova is the pacemaker, NeuroPace is the sentry. Their RNS (Responsive Neurostimulation) System is the world’s first “closed-loop” brain device. It doesn’t just blindly fire electricity; it listens. The device sits in the skull, monitoring brainwaves 24/7. It learns the specific electrical pattern that precedes a seizure, and then fires a micro-pulse to stop it before the patient even knows it’s happening.
The 2026 Catalyst: Profitability Inflection. For years, NeuroPace operated as a research-heavy organization. That shifted in late 2025 when the company posted its first-ever positive adjusted EBITDA quarter, validating the unit economics of the business. The 2026 roadmap focuses on the “Nautilus” project—a next-gen, smaller form factor designed to make cranial surgery less invasive. This product iteration is critical for moving the therapy earlier in the treatment paradigm.
CVRx, Inc. (NASDAQ: CVRX)
CVRx is proving that neurotech isn’t just for the brain—it’s for the heart. They developed Barostim, a device that electrically tricks the brain into relaxing the heart’s blood vessels. It is the first neuromodulation therapy approved for heart failure.
Most heart failure patients are on a cocktail of beta-blockers and ACE inhibitors that leave them exhausted. Barostim uses the body’s own nervous system to reduce the heart’s workload without the chemical fatigue.
The 2026 Catalyst: Reimbursement Shift. The technology works, but the billing process has historically been complex. That ends on January 1, 2026. The American Medical Association has assigned Barostim “Category I” CPT codes. In plain English, this means doctors no longer have to navigate “experimental” billing codes to get paid. This administrative change removes the primary procedural hurdle for hospital procurement departments.
BrainsWay Ltd. (NASDAQ: BWAY)
Not everyone wants brain surgery. BrainsWay is the leader in non-invasive Deep Transcranial Magnetic Stimulation (Deep TMS). They built a patented helmet that shoots magnetic fields deep into the brain structures to rewire neural pathways.
They are already the only company with FDA clearance to treat smoking addiction and OCD. But they have quietly been building the most robust mental health platform in the sector.
The 2026 Catalyst: Adolescent Mental Health. In November 2025, the FDA cleared BrainsWay’s Deep TMS for treating severe depression in adolescents (ages 15-21). This is a difficult demographic to treat, as parents are hesitant to utilize heavy anti-depressants. BrainsWay now offers a drug-free alternative with regulatory clearance. 2026 represents the first full year of commercialization for this new indication.

Neurodiagnostics & Monitoring
You cannot treat what you cannot measure. For most of history, diagnosing a brain issue required a patient to be either seizing on the floor or shoved into a claustrophobic MRI tube for 45 minutes.
This creates a dangerous “blind spot.” Strokes, non-convulsive seizures, and traumatic brain injuries often happen when there is no neurologist in the room. By the time the expert arrives, the damage is permanent.
Neurotech stocks in this basket are solving the access problem. They are taking diagnostics out of the basement radiology suite and bringing them to the bedside, the ambulance, and the home.
Ceribell, Inc. (NASDAQ: CBLL)
Ceribell is solving the “silent seizure” problem. In the ICU, many patients have seizures that show no physical shaking (non-convulsive status epilepticus). Their brains are frying, but from the outside, they just look like they are sleeping.
Traditional EEGs take hours to set up and require a specialist to interpret. Ceribell built a headband that any nurse can apply in 5 minutes. It uses AI to convert brainwaves into sound—literally turning brain signals into a “stethoscope for the brain.” If the machine hears the “seizure sound,” the nurse knows to call the doctor immediately.
The 2026 Catalyst: Pediatric Standard of Care. In April 2025, the FDA cleared Ceribell’s Clarity algorithm for pediatric patients (ages 1+). This was a massive regulatory win, as diagnosing seizures in children is notoriously difficult and time-sensitive. While late 2025 was about initial hospital pilots, 2026 marks the widespread commercial rollout. We are watching for this to become the default protocol in pediatric ICUs, effectively doubling their addressable patient population.
Hyperfine, Inc. (NASDAQ: HYPR)
Hyperfine is doing to the MRI what the laptop did to the mainframe. The standard MRI machine weighs nearly 10,000 pounds, costs millions, and requires a shielded room cooled by liquid helium. Hyperfine’s Swoop system is a portable MRI that rolls on wheels, plugs into a standard wall outlet, and uses low-field magnets to image the brain at the patient’s bedside.
It isn’t trying to replace the high-power scanners for detailed surgery planning. It is the “triage” scanner—immediate imaging for stroke or trauma patients who are too unstable to move.
The 2026 Catalyst: Operating Room Entry. Hyperfine is currently moving beyond the ICU and into the Operating Room. In late 2025, they began enrolling patients in the PRISM PMR study to validate the Swoop system for neurosurgical workflows. The goal is to allow surgeons to scan a patient’s brain during or immediately after surgery to check for complications like bleeding, without leaving the OR. In 2026, as this data matures, Hyperfine could pivot from a “nice-to-have” ICU tool to a critical piece of surgical infrastructure.
Compumedics Limited (ASX: CMP)
Compumedics is a veteran in the sleep diagnostic space. Based in Australia, they have historically been a high-end equipment manufacturer for sleep labs. But the market is moving away from expensive hospital sleep studies and toward home testing.
Their answer is Somfit—a clinical-grade wearable sensor that patients stick to their forehead at home. Unlike a Fitbit or Apple Watch that guesses sleep stages based on movement, Somfit records actual brain signals (EEG), providing lab-quality data without the hospital stay.
The 2026 Catalyst: Somfit D US Launch. The company is launching Somfit D (a disposable version) in the US market in the first half of 2026. This form factor is specifically designed for high-volume distribution through pharmacies and primary care clinics. It changes the business model from selling capital equipment (one-off sales) to a recurring revenue “consumable” model. With reimbursement codes already established for home sleep testing, the barrier to entry for this disposable product is minimal.

Brain-Computer Interfaces (BCI)
This is the “final mile” of neurotech. If neuromodulation is about nudging the nervous system, BCI is about connecting it. These companies aim to dissolve the barrier between biology and technology. That includes reading neural intentions (decoding thoughts) and writing neural commands (restoring movement).
Among neurotech stocks, this segment captures the most headlines—thanks to Neuralink (the private bellwether)—but it also carries the highest technical risk.
ONWARD Medical (Euronext: ONWD; OTCQX: ONWRY)
ONWARD is building the “Digital Bridge” for paralysis. When a spinal cord is severed, the brain can still issue commands (“move my leg”), but the signal hits a dead end at the injury site. ONWARD’s technology bridges that gap.
Their platform uses targeted stimulation to re-awaken the dormant spinal cord below the injury. In November 2025, they received FDA 510(k) clearance for their external ARC-EX system for home use. This was a pivotal moment: it moved spinal cord therapy from the clinic to the living room, allowing patients to regain hand strength and function through daily training.
The 2026 Catalyst: Empower BP. While the external device drives current revenue, the real value lies in the ARC-IM implant. ONWARD has secured an FDA Investigational Device Exemption (IDE) to run the “Empower BP” pivotal study. This trial isn’t just about walking; it targets blood pressure regulation—the number one “silent killer” and quality-of-life issue for quadriplegics. In 2026, the company shifts focus to enrolling and executing this trial. Positive data here would unlock a standard-of-care designation for spinal cord injuries that currently has zero approved treatments.
NeuroOne Medical Technologies (NASDAQ: NMTC)
If you want to connect a computer to the brain, you need a wire. For decades, surgeons used clunky, hand-made platinum iridium wires that were thick and hard to manufacture. NeuroOne prints “thin-film” electrodes using processes similar to microchip manufacturing.
The result is a neural interface that is thinner, flexible, and capable of much higher resolution recording. They have already commercialized this for epilepsy monitoring (sEEG), but the bigger play is their evolution from listening to destroying.
The 2026 Catalyst: Pain Management Expansion. In August 2025, NeuroOne received FDA clearance for its OneRF Trigeminal Nerve Ablation System. This device allows surgeons to treat severe facial pain by heating and destroying the specific nerve fibers causing the agony, using the same thin-film probe they use to map the brain. 2026 will be the first full year of commercialization for this indication. Furthermore, the company plans to initiate first-in-human cases for a new lower back pain application (basivertebral nerve ablation) in early 2026, effectively moving the company from a niche epilepsy player to a broader pain management competitor backed by Zimmer Biomet’s distribution network.
Scalably Slowing the Silver Tsunami
The “Silver Tsunami” is a demographic inevitability that the current healthcare model cannot handle. We simply cannot drug our way out of the coming surge in neurological decline without drowning patients in side effects. The neurotech thesis argues that the only scalable path forward is transitioning from chemical pharmacology to bio-electronic medicine.
This watchlist provides a tiered approach to neurotech stocks. Neuromodulation (Inspire, LivaNova, NeuroPace, CVRx, BrainsWay) offers immediate exposure to FDA-approved revenue streams and the shift from pills to pulses. Diagnostics (Ceribell, Hyperfine, Compumedics) bring critical brain data to the bedside, the ambulance, and the home. Finally, the BCI & Implant players (ONWARD, NeuroOne) provide high-beta exposure to bridging the final gap between biological intent and digital action.